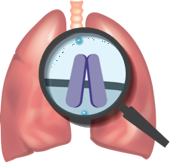
The underlying cause of cystic fibrosis (CF)
CF is caused by cystic fibrosis transmembrane conductance regulator (CFTR) protein defects. A mutation in the genes of a person with CF may make defective CFTR proteins that:
- Don’t open correctly
- Don’t get to the cell surface, where they are normally located
A person with CF may make CFTR proteins that have either or both of these defects.
Because of these defects, chloride ions cannot flow freely into or out of the cells as they should, leading to an imbalance of salt and water. This can lead to thick, sticky mucus in the lungs.
The underlying cause of cystic fibrosis (CF)
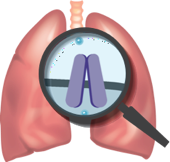
CF is caused by cystic fibrosis transmembrane conductance regulator (CFTR) protein defects. A mutation in the genes of a person with CF may make defective CFTR proteins that:
- Don’t open correctly
- Don’t get to the cell surface, where they are normally located
A person with CF may make CFTR proteins that have either or both of these defects.
Because of these defects, chloride ions cannot flow freely into or out of the cells as they should, leading to an imbalance of salt and water. This can lead to thick, sticky mucus in the lungs.
See how KALYDECO works
Watch This Video to See How KALYDECO Works
Learn how KALYDECO helps certain CFTR proteins work better.
View Important Safety Information and full Prescribing Information, including Patient Information, for KALYDECO.
KALYDECO targets the underlying cause

KALYDECO affects gating of the CFTR protein. KALYDECO is used in people age 1 month and older who have at least one mutation in their CF gene that is responsive to KALYDECO.
In patients with a mutation in their CF gene that is responsive to KALYDECO, KALYDECO works to help the "gates" stay open longer, allowing more chloride ions to move into and out of the cells. The movement of chloride ions may help keep a balance of salt and water in the lungs. KALYDECO does not increase the number of CFTR proteins at the cell surface.
What is known about how KALYDECO works was learned from studies conducted in a lab. Keep in mind that results from laboratory studies do not always match how these medicines work in a person. If you have any questions about your KALYDECO treatment, please speak with your healthcare provider.
IMPORTANT SAFETY INFORMATION
Before taking KALYDECO, tell your doctor about all of your medical conditions, including if you:
- have liver or kidney problems
- are allergic to KALYDECO or any ingredients in KALYDECO. See the Patient Information for a list of ingredients
- are pregnant or plan to become pregnant. It is not known if KALYDECO will harm your unborn baby. You and your doctor should decide if you will take KALYDECO while you are pregnant
- are breastfeeding or planning to breastfeed. It is not known if KALYDECO passes into your breast milk. You and your doctor should decide if you will take KALYDECO while you are breastfeeding
What is KALYDECO® (ivacaftor)?
KALYDECO is a prescription medicine used for the treatment of cystic fibrosis (CF) in people aged 1 month and older who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to KALYDECO.
Talk to your doctor to learn if you have an indicated CF gene mutation.
It is not known if KALYDECO is safe and effective in children under 1 month of age.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
KALYDECO may affect the way other medicines work, and other medicines may affect how KALYDECO works. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Especially tell your doctor if you take:
- the antibiotics rifampin (RIFAMATE®, RIFATER®) or rifabutin (MYCOBUTIN®)
- seizure medicines such as phenobarbital, carbamazepine (TEGRETOL®, CARBATROL®, EQUETRO®), or phenytoin (DILANTIN®, PHENYTEK®)
- St. John’s wort
- antifungal medicines such as ketoconazole, itraconazole (SPORANOX®), posaconazole (NOXAFIL®), voriconazole (VFEND®), or fluconazole (DIFLUCAN®)
- antibiotics such as telithromycin, clarithromycin (BIAXIN®), or erythromycin (ERY-TAB®)
What should I avoid while taking KALYDECO?
- KALYDECO can cause dizziness in some people who take it. If you experience dizziness, do not drive or operate machines until symptoms improve
- Avoid food or drink containing grapefruit while you are taking KALYDECO
What are the possible side effects of KALYDECO?
KALYDECO can cause serious side effects, including:
- High liver enzymes in the blood, which have happened in people receiving KALYDECO. Your doctor will do blood tests to check your liver:
- before you start KALYDECO
- every 3 months during your first year of taking KALYDECO
- every year while you are taking KALYDECO
For people who have had high liver enzymes in the past, your doctor may do blood tests to check the liver more often.
Call your doctor right away if you have any of the following symptoms of liver problems:
- pain or discomfort in the upper right stomach (abdominal) area
- yellowing of your skin or the white part of your eyes
- loss of appetite
- nausea or vomiting
- dark, amber-colored urine
- Serious allergic reactions have happened to people who are treated with KALYDECO. Call your healthcare provider or go to the emergency room right away if you have symptoms of an allergic reaction. Symptoms of an allergic reaction may include:
- rash or hives
- tightness of the chest or throat or difficulty breathing
- light-headedness or dizziness
- Abnormality of the eye lens (cataract), which has happened in some children and adolescents receiving KALYDECO. Your doctor should perform eye examinations before and during treatment with KALYDECO to look for cataracts.
The most common side effects of KALYDECO include:
- headache
- upper respiratory tract infection (common cold), including:
- sore throat
- nasal or sinus congestion
- runny nose
- stomach (abdominal) pain
- diarrhea
- rash
- nausea
- dizziness
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of KALYDECO. For more information, ask your doctor or pharmacist.
Use of KALYDECO in children aged 1 month to less than 6 months born from a pregnancy lasting (gestational age) less than 37 weeks has not been evaluated.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
For further information, please see full Prescribing Information, including Patient Information.
People with CF pictured may or may not be taking KALYDECO.